The Role of Hyaluronan in Pancreatic Cancer Biology and Therapy: Once Again in the Spotlight
Abstract
Pancreatic ductal adenocarcinoma (PDAC) is characterised by dense desmoplasia and hypoxic microenvironment. Our previous reports demonstrated that hyaluronan (HA), especially depression-molecular-weight HA, provides a favourable microenvironment for PDAC progression. However, the effect of hypoxia on HA metabolism remains unknown. Using quantitative real-time RT-PCR and western absorb analysis, we analysed the changes in the expression of HA-synthesizing enzymes (HAS2 and HAS3) and HA-degrading enzymes (HYAL1, KIAA1199/CEMIP) in PDAC cell lines under hypoxic atmospheric condition. Hypoxia increased the mRNA and protein expression of KIAA1199, whereas it decreased HYAL1 expression. The expression of HAS3 was increased and HAS2 remained unchanged in response to hypoxia. The upshot of KIAA1199 on hypoxia-induced cell migration was determined using a transwell migration assay and small-interfering RNA (siRNA). Hypoxia enhanced the migratory ability of PDAC cells, which was inhibited by KIAA1199 knockdown. We also used immunohistochemistry to analyse the protein expression of hypoxia inducible cistron (HIF) 1α and KIAA1199 in PDAC tissues. There was a meaning immunohistochemically positive correlation betwixt KIAA1199 and HIF1α. These findings advise that hypoxia-induced KIAA1199 expression may contribute to enhanced motility in PDAC.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of the deadliest diseases worldwide, with the lowest survival rate among all cancer typesane. PDAC is characterised by a dumbo desmoplastic stroma enriched with hyaluronan (HA)2,3.
HA is produced by HA-synthesizing enzymes (HASs) and is degraded into smaller fragments by hyaluronidases (HYALs)four,5,6. HA plays a disquisitional role in a multifariousness of cancerous behaviours including cell proliferation, migration, invasion, metastasis, angiogenesis, and resistance to drug delivery7,eight,9,10,11,12. In particular, low-molecular-weight HA (LMW-HA, 100 kDa or less) has been suggested to be essential in cancer progressionxiii,xiv.
KIAA1199 has been newly identified every bit an enzyme involved in HA degradation. Previous studies accept shown that KIAA1199 is overexpressed in various cancers, and is associated with an aggressive phenotype15,xvi,17,18,xix. Notably, KIAA1199 enhances the motion of colon cancer cells and is therefore called the cell migration-inducing protein (CEMIP). Nosotros accept too demonstrated previously that KIAA1199 expression correlates with poor prognosis in PDAC, and that forced expression of KIAA1199 increases the migration of PDAC cells20.
Hypoxia is one of the most common features in the neoplasm microenvironment21. Previous reports have shown that the average oxygenation in PDAC was markedly lower than that in normal tissue (the median pO2 of tumours was between 0 and v.3 mmHg, whereas the median pO2 of normal side by side tissue was between 9.3 and 92.7 mmHg22. Tumour growth results in hypoxia and hypoxia itself plays an important role in accelerating tumour progression, malignancy, and treatment resistance. Hypoxia signalling is mediated by hypoxia-inducible factors (HIFs), which are stabilised under hypoxic conditions23.
Increased HIF-1α and/or HIF-2α levels in biopsied specimens are associated with an increased mortality risk in some cancer types including PDAC24. Although many signalling pathways including mitogen-activated protein kinase (MAPK) signalling25, are known to be altered by hypoxic conditions in PDAC cells25, the effect of hypoxia on HA metabolism is unknown. In the present study, we investigated the relationship between hypoxia and HA metabolism in PDAC cells.
Materials and methods
Cell culture and reagents
Ii PDAC cell lines, BxPC3 and Panc-i (American Type Civilisation Collection, Manassas, VA, United states of america) were used in this study. PDAC cell lines were maintained in RPMI-1640 medium (Life Technologies, Grand Island, NY, USA) supplemented with x% foetal bovine serum (FBS) (Life Technologies) and 1% streptomycin and penicillin (Life Technologies) in a v% COii incubator at 37 °C. For hypoxic conditions, the cells were exposed to 1% Otwo using a depression oxygen culture kit (BIONIX low-oxygen culture kit, manufactured by Sugiyamagen Co., Ltd.) for 6–48 h. All experiments were repeated 3 times.
Quantitative real-time RT-PCR
Full RNA was isolated from jail cell lines using the RNeasy Mini Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's protocol. Start strand cDNA was synthesised from one.0 μg of total RNA using a SuperScript VILO cDNA synthesis Kit and Master Mix (Thermo Fisher Scientific Inc., Waltham, MA, U.s.a.). Real-time mRNA expression assay of HA-related genes (HAS2, HAS3, HYAL1, and KIAA1199), carbonic anhydrase 9 (CA9) and VEGF as a positive internal control and a housekeeping gene (β-actin and HPRT1) was performed using TaqMan @ Factor Expression Assays on the Step I Plus real-fourth dimension PCR organization (Thermo Fisher Scientific Inc.) according to the manufacturer's instructions. The assay numbers for these genes were as follows: Hs00193435_m1(HAS2), Hs00193436_m1 (HAS3), Hs00201046_m1 (HYAL1), Hs01552124_m1 (KIAA1199), Hs00154208_m1 (CA9), Hs80900055_m1 (VEGFA), Hs00194899_m1 (β-actin) and Hs02800695_m1 (HPRT1). Relative quantification was performed using Ct values to determine the expression for target genes and an internal control factor in all samples26.
Western absorb analysis
The cells were harvested and total protein was extracted with PRO-PREP protein extraction solution (iNtRON Biotechnology, Gyeonggi-do, Republic of korea), and poly peptide concentration was adamant using the bicinchoninic acid (BCA) protein analysis kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). Equal amounts of poly peptide were separated on a 4–15% Mini-PROTEAN Precast Gel (Bio-Rad, Philadelphia, PA, United states of america) and transferred onto PVDF membranes (ATTO, Tokyo, JAPAN). Membranes were blocked for 1 h with i% non-fatty milk in TBST buffer at room temperature, then incubated with antibodies confronting KIAA1199 (Proteintech Group, Rosemont, IL, Us) and β-actin (Proteintech Grouping) overnight at 4 °C or 1 h at room temperature, followed by incubation with secondary antibodies (Proteintech Grouping) for i h at room temperature. The proteins were visualised using an ECL Western Blotting Detection System (GE Healthcare, Buckinghamshire, England)26. To quantify the western blotting data, the intensities of proteins of interest in each gel were firstly normalized to their respective actin intensities, and so the normalized intensities were compared with the intensity of the control group and expressed every bit relative values to their controls27.
Cell migration analysis
The migratory ability of cells was determined using a transwell jail cell migration analysis with cell culture inserts containing a filter membrane with viii μm pores (BD Biosciences, Franklin Lajes NJ). The lower chamber was filled with RPMI1640 containing 10% foetal bovine serum (FBS). The upper chamber was filled with 1.0 × teniv cells (for Panc-1) or two.0 × 104 cells (for BxPC3) in RPMI RPMI1640 containing 1% FBS. Later on 48 h of incubation in normoxic and 1% Oii conditions, the cells remaining on the upper side of the filters were removed. The cells on the lesser surface of the membrane were stained with haematoxylin and eosin, and the number of cells that had migrated to the bottom surface of the membrane were counted in five randomly selected microscopic fields for each sample26.
siRNA targeting for KIAA1199
The siRNA for KIAA1199 (ON-TARGET plus SMART pool Human KIAA1199 L022291-00) and negative control siRNA (ON-TARGET plus Control siRNA Non-Targeting siRNA #1 D-001810-01-05) were purchased from GE Healthcare (Buckinghamshire, England). PDAC cells (Panc-1 and BxPC3) were transfected with 100 nM siRNA using DharmaFECT i Transfection Reagent (GE Healthcare) according to the manufacturer's instructions. After 48 h of treatment, the cells were immediately used for farther experiments26.
Immunohistochemistry
Formalin-fixed and paraffin-embedded sections were stained for KIAA1199 (1:100; Proteintech Grouping, Inc., Chicago, IL, United states of america) and HIF1α (1:50; Novus Biologicals, Inc. Littleton, CO, USA). EnVision + System- HRP Labelled Polymer Anti-Rabbit (Dako North America, Inc., Carpenteria, CA, USA) was used as the secondary antibody for anti-KIAA1199, and stained with diaminobenzidine (Liquid DAB + Substrate Chromogen Organisation; Dako North America, Inc.). Secondary antibody staining for anti-HIF1α was performed using the streptavidin–biotin technique using a SAB-PO(M) kit (NICHIREI BIOSCIENCES INC, Nippon). Sections were counterstained with haematoxylin, dried, and mounted. The proportion of positive cells was evaluated throughout the tissue department and was graded as follows: 0 (< 11%), 1 (eleven–40%), 2 (41–70%), or 3 (> seventy%). Staining intensity was graded equally 1 (weak), 2 (moderate), or 3 (potent). The total score was obtained past multiplying the positive proportion score with the intensity score. We and then divided all cases into 2 groups as the high expression group (with a score ranging from 3 to nine) and low expression grouping (with a score ranging from 0 to 2)20.
Ethics statement
Written informed consent was obtained from all patients whose tissues were used in this study. The study was canonical by the Institutional Review Board of the University of Occupational and Environmental Health, Kitakyushu, Nihon. All methods were performed in accord with the relevant guidelines and regulations.
Statistical assay
All statistical analyses were performed using STATA15 software (StataCorp. 2017.Stata Statistical Software: Release fifteen. College Station, TX: StataCorp LLC. Student's t-test and the chi-squared test were used for group comparison. Statistical significance was set at p < 0.05.
Results
Changes in the expression of HA-synthesizing and degrading enzymes in PDAC prison cell lines under normoxic and hypoxic conditions
Changes in the mRNA expression of HA-synthesizing enzymes (HAS2 and HAS3) and HA-degrading enzymes (HYAL1, KIAA1199) upon hypoxia were examined. In BxPC3 cells, KIAA1199 was markedly increased nether hypoxia (> 3.v-fold increased at 6 h), whereas the expression of HYAL1 was decreased under hypoxia (< 0.5-fold decreased from 6 to 48 h). HAS3 was enhanced under hypoxia (Fig. 1a). In Panc-1 cells, the expression of KIAA1199 was also significantly increased under hypoxia from 24 to 48 h (> 3.5-fold increased at 48 h), and the expression of HYAL1 was significantly decreased (< 0.5-fold decreased from 12 to 48 h). HAS3 was also enhanced nether hypoxic status (Fig. 1b). To confirm hypoxic condition in our experiments, nosotros examined mRNA expression of CA9 and VEGF, well-recognized HIF target. CA9 and VEGF were significantly increased in response to hypoxia in both cell lines.
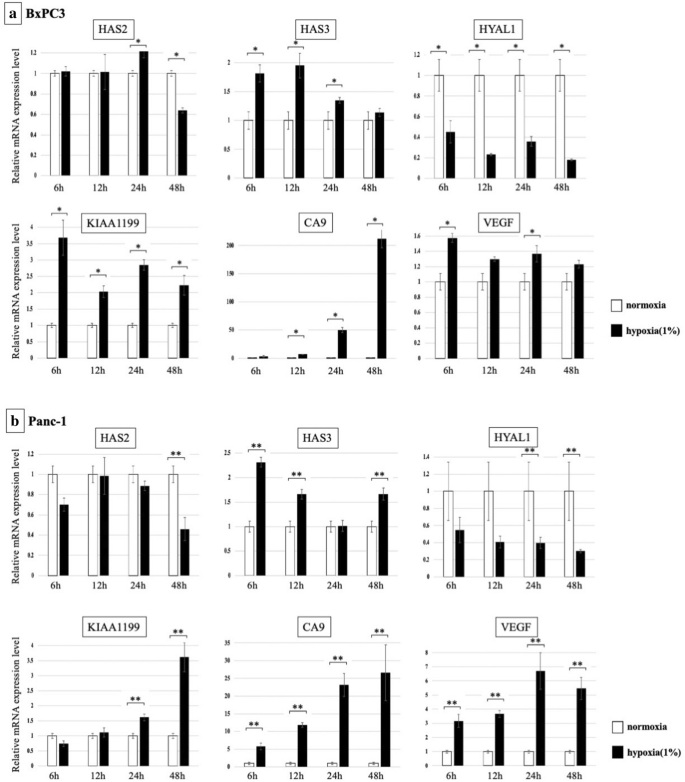
Changes in mRNA expression of HA synthesizing enzymes and HA degrading enzymes are shown. (a) BxPC3 cells: Expression of KIAA1199 and HAS3 was markedly increased under hypoxia, whereas HYAL1 was decreased (*p < 0.05). (b) Panc-one cells: Expression of KIAA1199 was significantly increased under hypoxia from 24 to 48 h, and HYAL1 was significantly decreased. HAS3 was also enhanced nether hypoxic condition (**p < 0.05).
The changes in the protein expression of KIAA1199 under hypoxia in BxPC3 cells was analysed using western blot assay. KIAA1199 poly peptide expression was increased in a time-dependent mode under hypoxic conditions (Fig. two and Fig.S1). β-actin levels were used equally the loading controls.
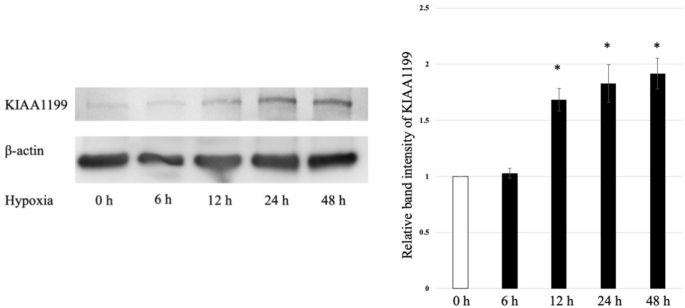
KIAA1199 protein increased in a time-dependent mode under hypoxic atmospheric condition. β-actin levels were used as loading controls. *p < 0.05 vs. normoxic condition.
Migratory ability of PDAC cells under hypoxic weather condition
We investigated the migratory ability of PDAC cells, Panc-i and BxPC3, under hypoxic conditions. In both jail cell lines, migratory ability was increased under hypoxic conditions (Fig. 3). The migratory ability of Panc-1 cells was increased upward to 400% and BxPC3 cells was up to 350% under hypoxic conditions.
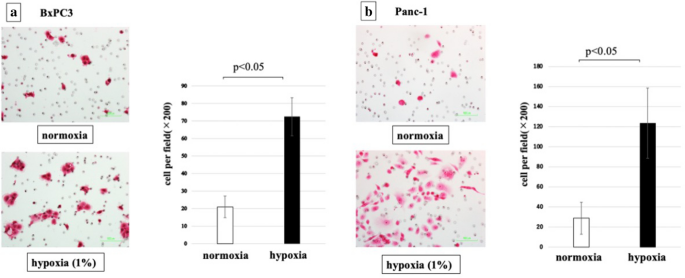
Jail cell migratory power of PDAC cells was significantly increased by hypoxia (p < 0.05). (a) BxPC3 cells, (b) Panc-1 cells.
Effect of KIAA1199 on PDAC cell migration in hypoxic condition
Our previous studies have shown that KIAA1199 strongly stimulates the migration of PDAC cells26. Therefore, we hypothesised that increased expression of KIAA1199 may play a role in the enhanced migration of PDAC cells upon hypoxia. To test this, we used siRNA to knock down KIAA1199 in Panc-1 and BxPC3 cells and examined their migration under hypoxic atmospheric condition (Fig. iv). Real-time RT-PCR showed that transfection with siRNA targeting for KIAA1199 (siRNA-KIAA1199) resulted in approximately seventy% knockdown. KIAA1199 knockdown significantly inhibited the hypoxia-induced increase in PDAC cell migration in both jail cell lines (p < 0.05).
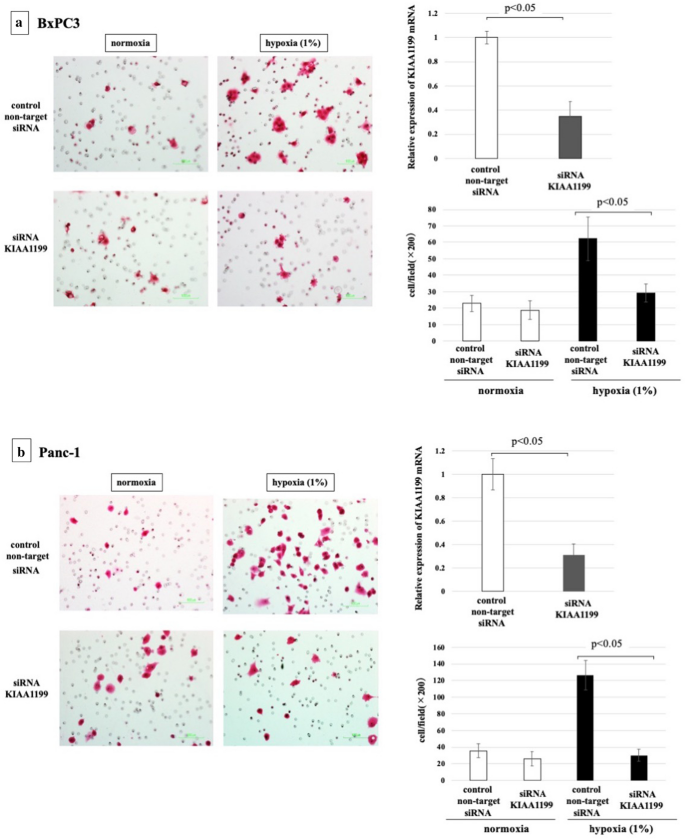
Cell migratory ability of PDAC cells under hypoxia with/without knockdown of KIAA1199. (a) BxPC3 cells, (b) Panc-1 cells. Real-time RT-PCR showed that transfection with siRNA targeting for KIAA1199 (siRNA-KIAA1199) resulted in approximately lxx% knockdown. Knockdown of KIAA1199 inhibited the increased migration by hypoxia (p < 0.05).
Immunohistochemical analysis of KIAA1199 and HIF1α in PDAC
We and so examined the human relationship between KIAA1199 and HIF1α expression, as a marker of hypoxia, using immunohistochemical analysis of archival tissues from 25 patients with PDAC. Representative immunostaining results are shown in Fig. 5 and Supplementary Figure S2. KIAA1199 expression was found to be high in 13 patients (52%), and HIF1α was highly expressed in 14 patients (56%). There was a pregnant positive correlation between KIAA1199 and HIF1α expression in our patient group (p < 0.05) (Table 1).
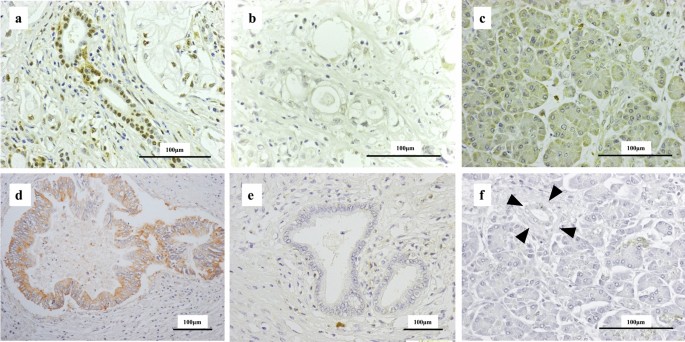
Immunostaining of PDAC tissues using anti-HIF1α antibody and anti-KIAA1199 antibody. (a) A representative image of strong nuclear labelling in PDAC cells (classified equally high HIF1α expression group). (b) A representative image classified as the depression HIF1α expression group. (c) Acinar cells with weak cytoplasmic labelling for HIF1α. (d) A representative image of potent cytoplasmic labelling of PDAC cells (classified as the high KIAA1199 expression group). (e) A representative image classified equally the low KIAA1199 expression group. (f) Acinar cells and noncancerous duct with no labelling for KIAA1199 (arrow head).
Discussion
In the present study, the changes in HA metabolism in PDAC jail cell lines under hypoxic atmospheric condition were investigated. The major findings were as follows: (1) Hypoxia increased the mRNA and protein expression of KIAA1199 in PDAC cells. (2) Hypoxia enhanced the migration of PDAC cells, whereas knockdown of KIAA1199 inhibited the migration enhanced by hypoxia. (3) There was a significant immunohistochemically positive correlation between HIF1α and KIAA1199 in PDAC tissues.
In our previous studies, we showed that HA metabolism was accelerated in PDAC cells10,28,29,30. Nonetheless, the expression patterns of HA-synthesizing or degradation enzymes under hypoxia in PDAC have been poorly characterised. In this study, nosotros demonstrated, for the first time, that hypoxia markedly increased the expression of KIAA1199, which is known to be involved in the malignant behaviour of cancer cells, in PDAC cells. These findings propose that changes in HA metabolism under hypoxia are associated with the malignant phenotype of PDAC cells.
Previous studies have revealed the mechanisms by which KIAA1199 promotes cancer progression. For example, KIAA1199 promotes the evolution of ovarian cancer past regulating PI3K/AKT signalling31. KIAA1199 regulates the proliferation and migration of vascular smooth muscle cells (VSMCs) in atherosclerosis by activating the Wnt-β-catenin signalling pathway32. Furthermore, KIAA1199 is known to increase the migration of cancer cells partly by mediating endoplasmic reticulum calcium leakage, which results in protein kinase Cα activation33. In our previous studies, knockdown of KIAA1199 expression was found to upshot in decreased migration, whereas forced expression of KIAA1199 increased the migration, invasion, and proliferation of PDAC cells20,26. In the present study, nosotros as well demonstrated that knockdown of KIAA1199 inhibits enhanced migration under hypoxia, suggesting the possible involvement of KIAA1199 in the ambitious phenotype nether hypoxia. Our present results and the previous findings may provide a rationale for developing novel therapeutic interventions targeting KIAA1199 for treating PDAC in the hereafter.
Our previous studies identified a novel phenotype, HAMP (hyaluronan-activated metabolism phenotype), characterized by activation of multiple HA metabolism cascades, in PDAC34. HAMP was divers as the increased expression of multiple HA metabolism genes, including KIAA1199. Yet, the mechanisms underlying the increased expression of these HA metabolism genes in PDAC are unclear. Ane such mechanism could be the neoplasm microenvironment (or tumour-stromal interactions) in PDAC. Our nowadays results propose that hypoxia could contribute to the development of the HAMP phenotype by increasing the expression of KIAA1199. Farther studies are thus needed to elucidate the relationship between hypoxia and HAMP in PDAC.
In determination, our present written report suggests that hypoxia-induced KIAA1199 expression may contribute to enhanced move and thus microenvironment-mediated cancer progression in PDAC.
Data availability
The datasets generated and/or analysed during the current written report are bachelor from the corresponding author upon reasonable request.
References
-
Vincent, A., Herman, J., Schulick, R., Hruban, R. H. & Goggins, M. Pancreatic cancer. Lancet 378, 607–620. https://doi.org/ten.1016/s0140-6736(10)62307-0 (2011).
-
Mahlbacher, V., Sewing, A., Elsässer, H. P. & Kern, H. F. Hyaluronan is a secretory production of human pancreatic adenocarcinoma cells. Eur. J. Cell Biol. 58, 28–34 (1992).
-
Whatcott, C. J. et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res. 21, 3561–3568. https://doi.org/10.1158/1078-0432.Ccr-14-1051 (2015).
-
Itano, Due north. & Kimata, Grand. Mammalian hyaluronan synthases. IUBMB Life 54, 195–199. https://doi.org/10.1080/15216540214929 (2002).
-
Stern, R. Hyaluronan catabolism: a new metabolic pathway. Eur. J. Cell Biol. 83, 317–325. https://doi.org/10.1078/0171-9335-00392 (2004).
-
Toole, B. P. Hyaluronan: from extracellular glue to pericellular cue. Nat. Rev. Cancer 4, 528–539. https://doi.org/10.1038/nrc1391 (2004).
-
Itano, N., Zhuo, L. & Kimata, Thousand. Impact of the hyaluronan-rich tumor microenvironment on cancer initiation and progression. Cancer Sci. 99, 1720–1725. https://doi.org/10.1111/j.1349-7006.2008.00885.x (2008).
-
Jacobetz, Yard. A. et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120. https://doi.org/10.1136/gutjnl-2012-302529 (2013).
-
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates concrete barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Prison cell 21, 418–429. https://doi.org/10.1016/j.ccr.2012.01.007 (2012).
-
Sato, N., Kohi, S., Hirata, K. & Goggins, M. Role of hyaluronan in pancreatic cancer biology and therapy: One time once more in the spotlight. Cancer Sci. 107, 569–575. https://doi.org/10.1111/cas.12913 (2016).
-
Sironen, R. Yard. et al. Hyaluronan in human malignancies. Exp. Cell Res. 317, 383–391. https://doi.org/x.1016/j.yexcr.2010.11.017 (2011).
-
Toole, B. P., Wight, T. North. & Tammi, M. I. Hyaluronan-cell interactions in cancer and vascular disease. J. Biol. Chem. 277, 4593–4596. https://doi.org/ten.1074/jbc.R100039200 (2002).
-
Sugahara, K. North. et al. Tumor cells heighten their ain CD44 cleavage and motility by generating hyaluronan fragments. J. Biol. Chem. 281, 5861–5868. https://doi.org/x.1074/jbc.M506740200 (2006).
-
Wu, M. et al. A novel role of low molecular weight hyaluronan in breast cancer metastasis. Faseb. J. 29, 1290–1298. https://doi.org/x.1096/fj.fourteen-259978 (2015).
-
Birkenkamp-Demtroder, Thousand. et al. Repression of KIAA1199 attenuates Wnt-signalling and decreases the proliferation of colon cancer cells. Br. J. Cancer 105, 552–561. https://doi.org/ten.1038/bjc.2011.268 (2011).
-
Evensen, N. A. et al. Hypoxia promotes colon cancer dissemination through upwards-regulation of jail cell migration-inducing protein (CEMIP). Oncotarget 6, 20723–20739. https://doi.org/x.18632/oncotarget.3978 (2015).
-
Fink, Due south. P. et al. Consecration of KIAA1199/CEMIP is associated with colon cancer phenotype and poor patient survival. Oncotarget 6, 30500–30515. https://doi.org/10.18632/oncotarget.5921 (2015).
-
Kuscu, C. et al. Transcriptional and epigenetic regulation of KIAA1199 gene expression in human breast cancer. PLoS One seven, e44661. https://doi.org/ten.1371/journal.pone.0044661 (2012).
-
Matsuzaki, S. et al. Clinicopathologic significance of KIAA1199 overexpression in human being gastric cancer. Ann. Surg. Oncol. 16, 2042–2051. https://doi.org/ten.1245/s10434-009-0469-6 (2009).
-
Koga, A. et al. KIAA1199/CEMIP/HYBID overexpression predicts poor prognosis in pancreatic ductal adenocarcinoma. Pancreatology 17, 115–122. https://doi.org/10.1016/j.pan.2016.12.007 (2017).
-
Brahimi-Horn, M. C., Chiche, J. & Pouysségur, J. Hypoxia and cancer. J. Mol. Med. (Berl.) 85, 1301–1307. https://doi.org/ten.1007/s00109-007-0281-3 (2007).
-
Koong, A. C. et al. Pancreatic tumors show high levels of hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 48, 919–922. https://doi.org/10.1016/s0360-3016(00)00803-eight (2000).
-
Yuen, A. & Díaz, B. The touch of hypoxia in pancreatic cancer invasion and metastasis. Hypoxia (Auckl) ii, 91–106. https://doi.org/ten.2147/hp.S52636 (2014).
-
Semenza, G. L. Defining the part of hypoxia-inducible factor i in cancer biology and therapeutics. Oncogene 29, 625–634. https://doi.org/10.1038/onc.2009.441 (2010).
-
Jia, Y. et al. Phosphorylation of 14–three-3ζ links YAP transcriptional activation to hypoxic glycolysis for tumorigenesis. Oncogenesis eight, 31. https://doi.org/ten.1038/s41389-019-0143-1 (2019).
-
Kohi, Due south., Sato, N., Koga, A., Matayoshi, N. & Hirata, K. KIAA1199 is induced by inflammation and enhances malignant phenotype in pancreatic cancer. Oncotarget 8, 17156–17163. https://doi.org/x.18632/oncotarget.15052 (2017).
-
Zhang, X. et al. Dual effects of arsenic trioxide on tumor cells and the potential underlying mechanisms. Oncol. Lett. 16, 3812–3820. https://doi.org/10.3892/ol.2018.9086 (2018).
-
Cheng, Ten. B., Kohi, South., Koga, A., Hirata, G. & Sato, N. Hyaluronan stimulates pancreatic cancer cell motion. Oncotarget 7, 4829–4840. https://doi.org/10.18632/oncotarget.6617 (2016).
-
Kohi, S. et al. A novel epigenetic mechanism regulating hyaluronan production in pancreatic cancer cells. Clin. Exp. Metastasis 33, 225–230. https://doi.org/10.1007/s10585-015-9771-9 (2016).
-
Sato, N., Cheng, X. B., Kohi, Due south., Koga, A. & Hirata, K. Targeting hyaluronan for the treatment of pancreatic ductal adenocarcinoma. Acta Pharm. Sin. B 6, 101–105. https://doi.org/10.1016/j.apsb.2016.01.002 (2016).
-
Shen, F. et al. CEMIP promotes ovarian cancer development and progression via the PI3K/AKT signaling pathway. Biomed. Pharmacother. 114, 108787. https://doi.org/10.1016/j.biopha.2019.108787 (2019).
-
Xue, Q., Wang, X., Deng, 10., Huang, Y. & Tian, West. CEMIP regulates the proliferation and migration of vascular smooth muscle cells in atherosclerosis through the WNT-beta-catenin signaling pathway. Biochem. Cell Biol. 98, 249–257. https://doi.org/ten.1139/bcb-2019-0249 (2020).
-
Evensen, N. A. et al. Unraveling the role of KIAA1199, a novel endoplasmic reticulum protein, in cancer prison cell migration. J. Natl. Cancer Inst. 105, 1402–1416. https://doi.org/ten.1093/jnci/djt224 (2013).
-
Kudo, Y., Kohi, Due south., Hirata, K., Goggins, M. & Sato, Due north. Hyaluronan activated-metabolism phenotype (HAMP) in pancreatic ductal adenocarcinoma. Oncotarget x, 5592–5604. https://doi.org/10.18632/oncotarget.27172 (2019).
Acknowledgements
Nosotros give thanks Ms. Ueda (Research Assistant, Department of Surgery, University of Occupational and Environmental Health, Kitakyushu, Japan) for providing technical assist. We would like to give thanks Editage (www.editage.com) for English linguistic communication editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Writer data
Affiliations
Contributions
T.O. carried out the molecular studies and drafted the manuscript. North.S. conceived the written report, participated in its design and coordination, and helped typhoon the manuscript. A.Thousand., Y.A., Y.K., T.A., and Southward.Yard. participated in the molecular studies. Thou.H. participated in the written report design. All authors take read and approved the terminal manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional data
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Data
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits utilise, sharing, adaptation, distribution and reproduction in any medium or format, equally long as you give appropriate credit to the original author(due south) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third political party material in this article are included in the article'due south Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Artistic Eatables licence and your intended employ is not permitted by statutory regulation or exceeds the permitted utilise, yous will need to obtain permission direct from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and Permissions
About this article
Cite this article
Oba, T., Sato, Due north., Adachi, Y. et al. Hypoxia increases KIAA1199/CEMIP expression and enhances jail cell migration in pancreatic cancer. Sci Rep eleven, 18193 (2021). https://doi.org/10.1038/s41598-021-97752-z
-
Received:
-
Accustomed:
-
Published:
-
DOI : https://doi.org/10.1038/s41598-021-97752-z
Comments
Past submitting a comment you hold to bide by our Terms and Community Guidelines. If you find something abusive or that does non comply with our terms or guidelines please flag it as inappropriate.
Source: https://www.nature.com/articles/s41598-021-97752-z
0 Response to "The Role of Hyaluronan in Pancreatic Cancer Biology and Therapy: Once Again in the Spotlight"
Post a Comment